Subdural Hematomas - Subacute and Chronic
Pathophysiology and Clinical Presentation:
Chronic subdural hematomas (cSDH) remain a relatively common intracranial pathologic condition with an incidence in the general population of approx. 1-5/100k patient years reported, with significantly increasing incidence of presentation in the elderly (>65-70 yo), rising to approximately 8.2-18.8/100k patients in older populations. The etiology of cSDH remains controversial and elusive, first described by Virchow in 1857 by Virchow as inflammatory response forming a membrane in the inner surface of the dura forming a hematoma. More recent studies and theories have suggested that often incidental, subclinical trauma may “seed” the hematoma (Phase 1), with gradual volume expansion and maturation of the hematoma secondary to inflammatory fibroblast proliferation, granulation tissue formation, and release of angiogenic factors within 3-4 weeks of the primary injury.
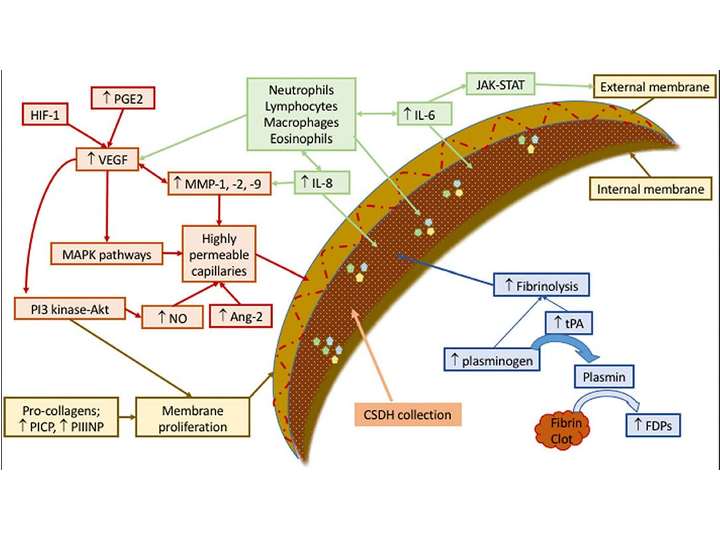
Figure 1. Schematic representation of mechanisms involved in development and sustenance of cSDH. Contributing factors have been labeled as green (recruiting inflammatory cell), red (forming permeable capillaries), brown (forming inner and outer membranes), and blue (ongoing hemorrhage due to fibrinolysis). Ang, angiopoietin; FDPs, fibrin/fibrinogen degradation products; HIF, hypoxia-inducible factor; IL, interleukin; JAK-STAT, Janus kinase-signal transducer and activator of transcription; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NO, nitric oxide; PGE, prostaglandin E; PI3-Akt, phosphatidylinositol 3-kinase-serine/threonine kinase; PICP, procollagen type 1; PIIINP, procollagen type 3; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor. Reproduced from (3,4) under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
It has been hypothesized that leakage from these inflammatory neomembrane capillaries, secondary to highly permeable endothelial gap junctions and microtears of the fragile neovascularized vessels contribute to this growth “latency” period (Phase 2), followed by clinical presentation (Phase 3) when a patient presents with symptoms (often non-localizing) including headaches, mental status changes, weakness, paresthesias, dysarthria, gait abnormalities, nausea, vomiting, stroke, seizures (10-20%), coma (2-15%), and brain herniation (approx. 2%) reported in many series and reviews. Risk factors that may be associated with and contribute to cSDH formation, growth, or recurrence include falls, antiplatelet or anticoagulation usage (10-30%), underlying systemic disease (i.e. Hepatic, Renal, Coagulaopathies), epilepsy, male gender, elderly age, and alcohol usage.[i],[ii],[iii],[iv]
cSDH Surgical Treatment Strategies:
The treatment and management of cSDH remains controversial and has traditionally been treated either conservatively with observation and serial imaging in asymptomatic patients and smaller collections (without midline shift) or more aggressively with surgical evacuation in symptomatic patients or larger collections (with greater than 5 mm of midline shift). There is a variety of surgical options for cSDH treatment including twist-drill craniotomy (5-10 mm incision) with or without a subdural evacuating port system (SEPS), burr hole evacuation (silver dollar or up to 30mm incision) with or without drains, or craniotomy (>30 mm incision). Irrespective of the type of evacuation, cSDH surgical treatment continues to have high recurrence rates ranging from 5% to 37%, thereby subjecting these patients to repeat surgical interventions, hospital admissions, and the associated complications including peri-operative morbidity, diminished neurologic and functional status, and even mortality[v].
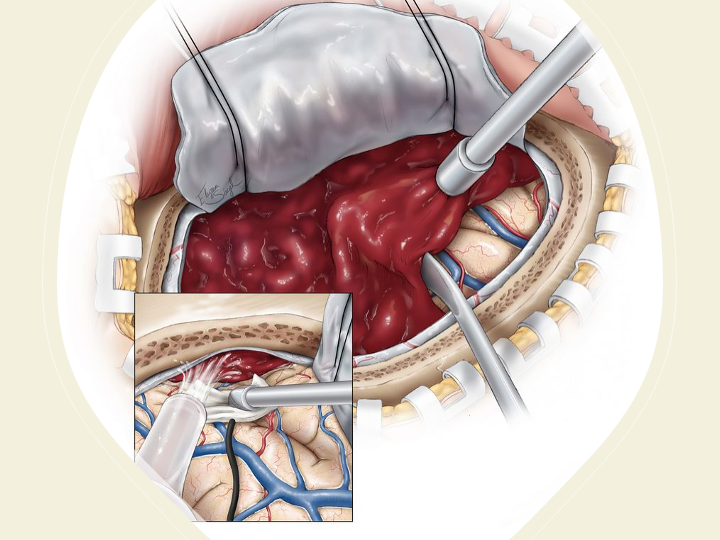
Figure 2. Surgical craniotomy for Subdural Hematoma. (https://www.neurosurgicalatlas.com/volumes/emergency-neurosurgery-and-trauma/traumatic-hematoma/acute-subdural-hematoma((c)2022, The Neurosurgical Atlas)[i]
Endovascular Treatment Strategies (Middle Meningeal Artery (MMA) Embolization):
Although conventional surgical methods, such as burr hole irrigation or observation in asymptomatic or minimally symptomatic patients have been the mainstay of treatment, middle meningeal artery (MMA) embolization has emerged as a promising adjunctive or alternative treatment. MMA embolization is a neuroendovascular technique which involves placing a microcatheter into the Middle Meningeal Artery, most commonly on the side of the cSDH (occasionally, bilateral embolization has been recommended for larger or bilateral collection. Embolization of both the anterior (frontal) and posterior (parietal) division is performed using a variety of embolic materials (liquid NBCA, Onyx, coils), although polyvinyl alcohol particles of <250 microns are most commonly used to achieve distal penetration and occlusion of the pre-capillary and capillary beds. The micro-leakage of blood and transudative proteinaceous inflammatory fluid in these inflammatory membranes is creating an imbalance in cycle of resorption and preventing resolution or promoting recurrence or growth of these cSDH collections and associated mass effect and midline shift.
[i] https://www.neurosurgicalatlas.com/volumes/emergency-neurosurgery-and-trauma/traumatic-hematoma/acute-subdural-hematoma (Contributor: Jonathan Weyhenmeyer, MD)
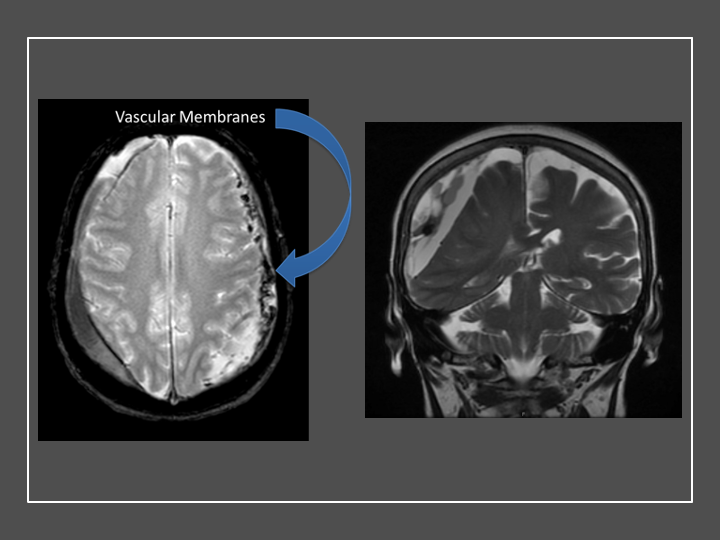
Figure 3. Neovascularized membranes within cSDH
Care must be taken to place the microcatheter within a safe position for embolization, with special attention to potential collateral branches to the orbit/eye via sphenoid or meningo-lacrimal branches (which may cause vision loss) or petrosal branches supplying the geniculate ganglion, the tympanic portion of the facial nerve, and often the trigeminal nerve via a branch coursing along the greater petrosal nerve. Once a safe position in the main trunk of the MMA, or superselectively in each of its divisions is achieved, embolization is performed until stasis of contrast and dense contrast staining of the dura is observed within the embolized vascular beds.[i]
[i] Shotar E, Premat K, Lenck S, Degos V, Marijon P, Pouvelle A, Pouliquen G, Mouyal S, Abi Jaoude S, Sourour NA, Mathon B, Clarençon F. Angiographic Anatomy of the Middle Meningeal Artery in Relation to Chronic Subdural Hematoma Embolization. Clin Neuroradiol. 2021 Feb 24. doi: 10.1007/s00062-021-00996-5. Epub ahead of print. PMID: 33625552.
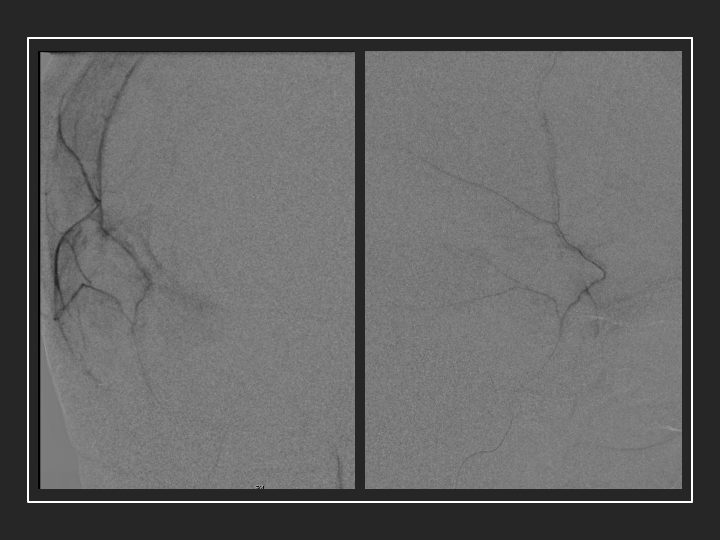
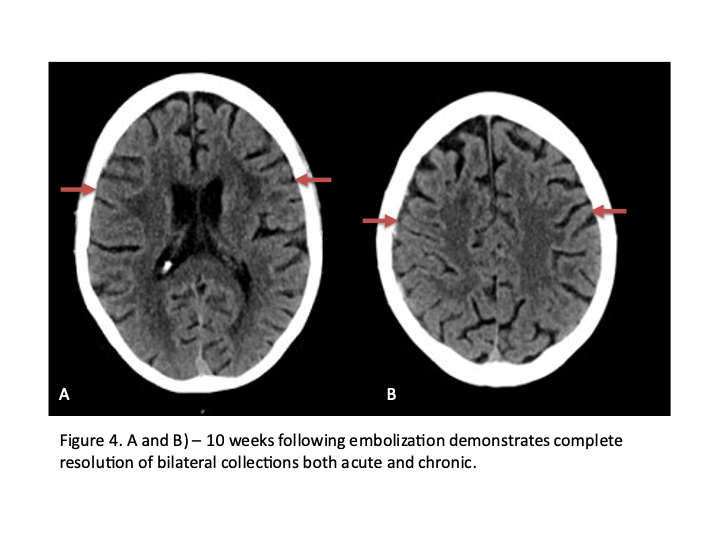
Figure 4. Dense contrast staining of vascular membranes during embolization.
Masaki Komiyama first introduced MMA embolization as a treatment option for recurrent cSDH in 1994, when he described employing it in combination with burr hole drainage in a traumatic patient with a linear skull fracture and recurrent hemorrhage.3 Over the last decade, there has been renewed interest in endovascular approaches to treating cSDH, secondary to the significant rate of recurrence, persistence, and complications associated with surgical only approaches. In the most recent and largest meta-analysis performed by Ironside etal., they reviewed a total of 20 studies comprising 1416 patients with cSDH, who met their inclusion criteria. Five double-arm studies (902 patients) compared outcomes with conventional management versus MMA embolization. Fifteen single-arm studies (514 patients) reported outcomes with or without surgical evacuation. In this review, MMA embolization was performed for recurrent cSDH after prior surgical evacuation in 47.8%, prophylactically after surgical evacuation in 23.2%, and primarily(upfront) in 28.4%. Embolization materials included particles (n=403), liquid embolics (n=143), coils (n=171), microspheres (n=86), and Onyx (n=80). The pooled recurrence rates (MMA vs conventional) were 4.8% (95% CI 3.2% to 6.5%) vs. 21.5% (0.6% to 42.4%), the surgical rescue rates, 4.4% (2.8% to 5.9%), vs 16.4% (5.9% to 27.0%), and in-hospital complication rates 1.7% (0.8% to 2.6%), vs 4.9% (2.8% to 7.1%). Compared with conservative management, MMA embolization was associated with lower rates of cSDH recurrence (OR=0.15 (95% CI 0.03 to 0.75), p=0.02) and surgical rescue (OR=0.21 (0.07 to 0.58), p=0.003). In-hospital complication rates were comparable between the two cohorts (OR=0.78 (0.34 to 1.76), p=0.55).
Conclusions:
Chronic Subdural Hematomas remain a challenging and common pathologic condition that is increasingly affecting our growing elderly population. Traditional treatment strategies of observation and surgical drainage remain effective tools for their treatment, however, the advent of MMA embolization has resulted in significant improvements in their management, decreasing recurrence, surgical rescue, and in-hospital mortality rates as shown in the early experience of many experienced centers and meta-analysis of retrospective and prospective single and double-arm studies. Prospective randomized studies to better evaluate this novel application of traditional techniques and their role are ongoing and planned.
References:
[1] Yang W, Huang J. Chronic Subdural Hematoma: Epidemiology and Natural History. Neurosurg Clin N Am. 2017 Apr;28(2):205-210. doi: 10.1016/j.nec.2016.11.002. Epub 2017 Feb 1. PMID: 28325454.
[1] Ironside N, Nguyen C, Do Q, Ugiliweneza B, Chen CJ, Sieg EP, James RF, Ding D. Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis. J Neurointerv Surg. 2021 Oct;13(10):951-957. doi: 10.1136/neurintsurg-2021-017352. Epub 2021 Jun 30. PMID: 34193592.
[1] Komiyama M, Yasui T, Tamura K, Nagata Y, Fu Y, Yagura H. Chronic subdural hematoma associated with middle meningeal arteriovenous fistula treated by a combination of embolization and burr hole drainage. Surg Neurol. 1994 Oct;42(4):316-9. doi: 10.1016/0090-3019(94)90400-6. PMID: 7974127.
[1] Moshayedi P, Liebeskind DS. Middle Meningeal Artery Embolization in Chronic Subdural Hematoma: Implications of Pathophysiology in Trial Design. Front Neurol. 2020 Aug 27;11:923. doi: 10.3389/fneur.2020.00923. PMID: 32973670; PMCID: PMC7481478.
[1] Xu CS, Lu M, Liu LY, Yao MY, Cheng GL, Tian XY, Xiao F, Wan Q, Chen F. Chronic subdural hematoma management: clarifying the definitions of outcome measures to better understand treatment efficacy – a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2017 Feb;21(4):809-818. PMID: 28272701.
[1] https://www.neurosurgicalatlas.com/volumes/emergency-neurosurgery-and-trauma/traumatic-hematoma/acute-subdural-hematoma (Contributor: Jonathan Weyhenmeyer, MD)
[1] Shotar E, Premat K, Lenck S, Degos V, Marijon P, Pouvelle A, Pouliquen G, Mouyal S, Abi Jaoude S, Sourour NA, Mathon B, Clarençon F. Angiographic Anatomy of the Middle Meningeal Artery in Relation to Chronic Subdural Hematoma Embolization. Clin Neuroradiol. 2021 Feb 24. doi: 10.1007/s00062-021-00996-5. Epub ahead of print. PMID: 33625552.

