Brain and Spine AVM
Title: Rapid Progression of Paralysis in a 40 yo Man from a CervicoMedullary Brain AV Fistula
Clinical Presentation:
A 40 yo Man presents to the Emergency Department with a Chief Complaint of abdominal pain. Initial evaluation with a CT of the Abdomen reveals a 3.8 x 3.7 cm pancreatic head/body mass with distal pancreas body/tail atrophy with associated splenic and superior mesenteric vein thrombosis. MRCP demonstrates a large neoplastic mass involves the posterior aspect of the proximal and mid pancreatic body and measures approximately 4.5 x 4.5 cm. The splenic vein is occluded with venous collaterals/varices noted along collapsed gastric fundus. The other mesenteric arteries and veins are patent. No evidence of bowel or biliary obstruction. No evidence of hepatic metastatic disease at presentation.
In addition to his abdominal symptoms, he presents with mild dysarthria and moderate weakness of the left arm and leg. Upon further inquiry, he reports experiencing paresthesias and numbness on the left side approximately 2 months prior, and mild clumsiness of the left side 1 month prior to presentation. Bowel and Bladder dysfunction were also observed on detailed evaluation. MRI of the Brain was performed demonstrating extensive abnormalities in the Right paramedian Pons extending inferiorly into the Medulla and Upper Cervical Spinal Cord with heterogeneous irregular enhancement. (Figures 1 and 2)
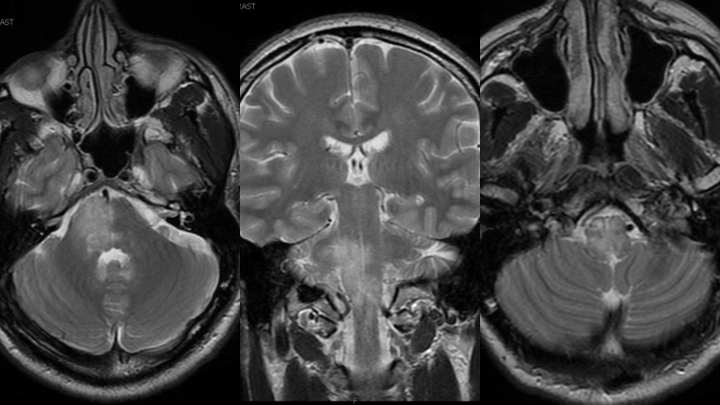
Figure 1. MRI T2 Axial and Coronal sequences demonstrating extensive hyperintensity within the right hemi-pons extending inferiorly into the medulla and cervical spinal cord.
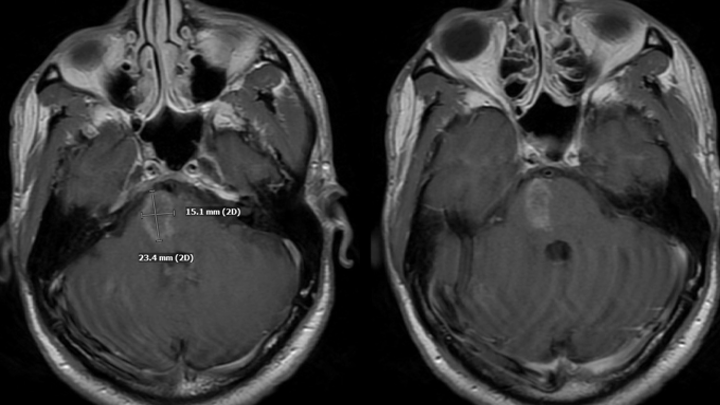
Figure 2. MRI post contrast images reveal heterogeneous, irregular enhancement within the lesion.
In a patient with a known Pancreatic Tumor, the differential diagnosis of potential CNS pathologies is extensive. Initial considerations included Metastatic Pancreatic Cancer (rare), Contemporaneous Brain Tumor, Paraneoplastic Syndromes, Metabolic/Autoimmune/Infection Syndromes, and Vascular Disease. Brain Biopsy and LP were considered, however, more careful evaluation of the MRI demonstrated extensive perimedullary brainstem and cervical cord enhancement suspicious for abnormal blood vessels. (Figure 3)

Figure 3. MRI post contrast demonstrated extensive perimedullary brainstem and cervical cord serpiginous enhancement pattern suspicious for abnormal blood vessels.
These findings necessitated a Cerebral Angiogram, which confirmed the diagnosis of a Cervical-Medullary Dural Arterio-Venous Fistula, supplied dominantly by the Neuromeningeal division of the right Ascending Pharyngeal Artery and secondarily from the right Posterior Meningeal Artery, with extensive superior venous drainage into the Perimesencephalic and Pontine systems and inferiorly into the Perimedullary venous drainage along the Cervical Spinal Cord resulting in the severe Venous Hypertension and Venous Ischemic injury observed on the initial MRI’s. (Figures 4 and 5)

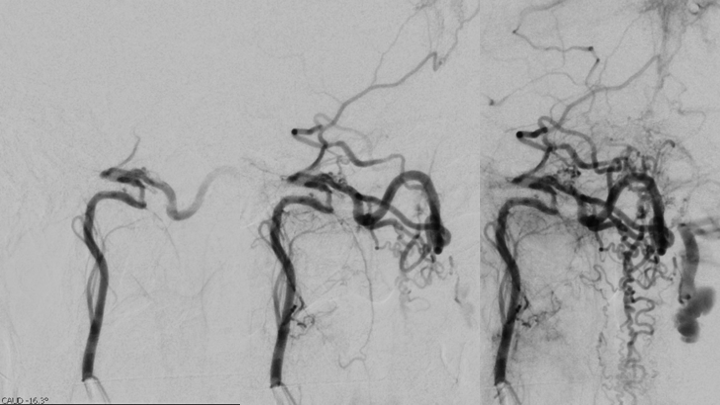
Figure 4. Catheter angiography demonstrates a High Flow and High Pressure A-V fistula of the Neuromeningeal division of the right Ascending Pharyngeal Artery (Red Arrow) with extensive Venous Drainage into the Antegrade Perimesencephalic and Retrograde Perimedullary systems of the Brainstem and Cervical Spinal Cord (Blue Arrows).

Figure 5. Additional Catheter angiography also suggested additional contributions to the A-V fistula from the right Posterior Meningeal Artery originating from the right Vertebral Artery.
Therapeutic Management:
Within the initial week of his evaluation, the patient developed progressive dysarthria and near hemiplegia of the left arm and leg, which prompted urgent treatment considerations to prevent further neurologic decline and potentially devastating effects of hemorrhage or infarct within the Brainstem and Cervical Cord. A multi-disciplinary staged plan was developed whereby we performed embolization of the dominant right Ascending Pharyngeal Artery contributions to eliminate the fistulas from this territory using Coil embolization across the fistulas. (Figures 6 and 7)
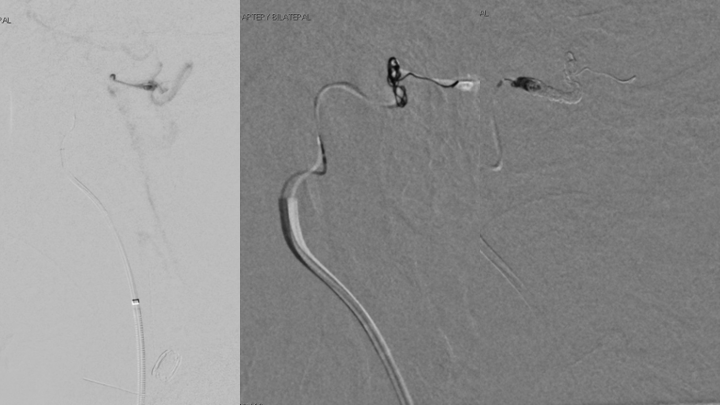
Figure 6. Trans arterial Coil embolization or the right Ascending Pharyngeal Artery contributions to eliminate the fistulas from this territory.
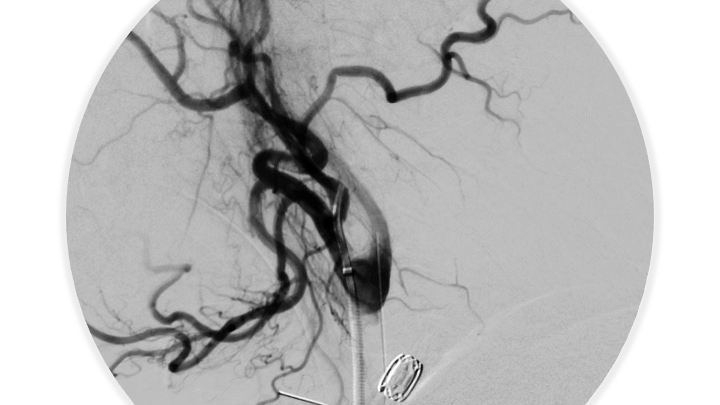
Figure 7. Post-embolization angiography confirms complete obliteration of the right ECA supply to the Fistula.
We then placed the patient on anti-coagulation to avoid uncontrolled Venous thrombosis and allowed the collateral venous fistulas from the right Posterior Meningeal Artery to mature for Stage 2 embolization. Follow-up Angiography confirms maturation of this supply (Figure 8) and demonstration of the entirety of the remaining fistulas (Figure 9), and Embolization was successfully performed with NBCA (liquid N-Butyl CyanoAcrylate) to completely eliminate all visible A-V fistulas without occluding critical venous drainage pathways (Figure 10).
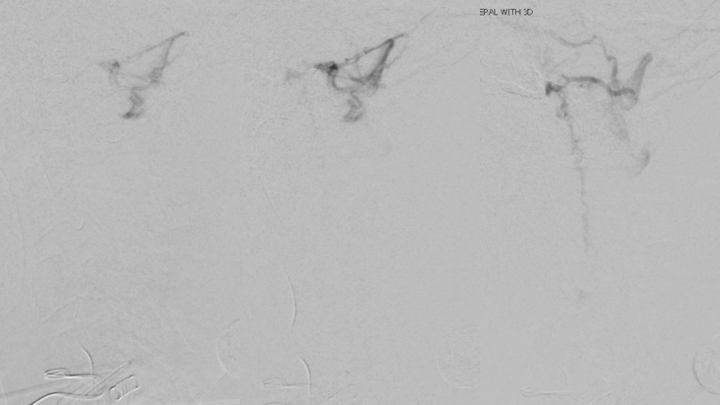
Figure 8. Follow-up Angiography demonstrating maturation of the right Posterior Meningeal contributions to the A-V fistula.
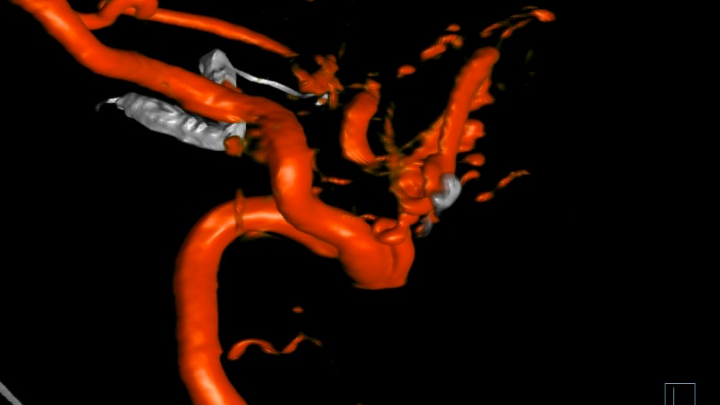
Figure 9. 3D Angiography demonstrates the residual A-V fistulous targets for embolization.
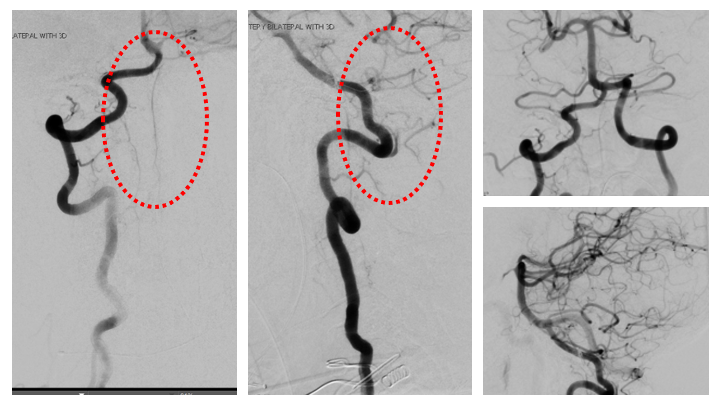
Figure 10. Complete cure of the A-V fistula is observed following NBCA embolization with preservation of the critical Arterial and Venous structures of the Brainstem and Spinal Cord.
Clinical Course:
Within 1 week of his therapy, he regained Bowel and Bladder function, experienced significant improvement in his Speech Fluency, and early signs of recovering motor function in LUE and LLE (from 0/5 to 1/5). His symptoms of Depression also began to improve as he now felt there was “Hope” and an opportunity to persevere and potentially recover from these devastating diseases. MRI performed 1 week after definitive treatment confirmed improving abnormalities within the Brainstem and Spinal Cord with resolution of the abnormal vasculature. (Figures 11 and 12).
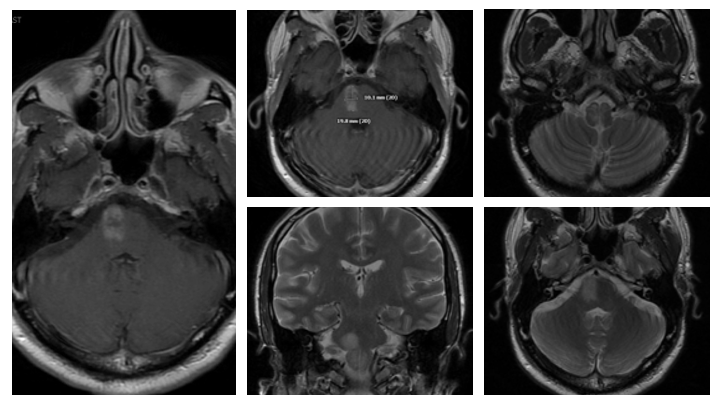
Figure 11. Followup MRI at 1 week demonstrates early improvement and decreased T2 signal, cerebral edema, and enhancement.
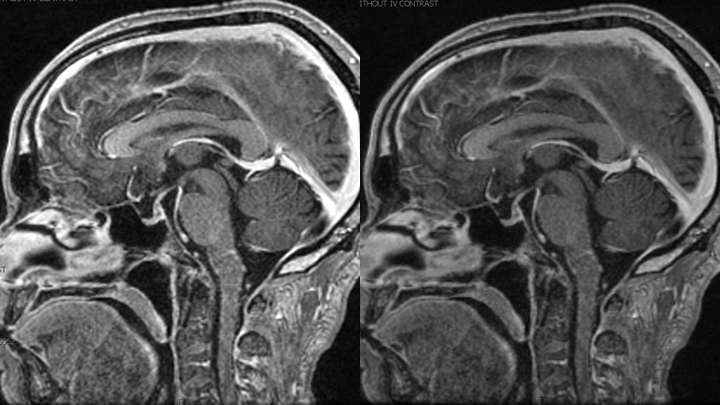
Figure 12. MRI demonstrates resolution of the abnormally enhancing vessels of the A-V fistula.
The patient was discharged to Subacute Rehabalitation, where after 1 month of recovery, he continues to regain mild strength with the LUE and LLE, near complete resolution of his dysarthria, complete recovery of bowel and bladder functions. He is scheduled to receive Chemotherapy for his Pancreatic Neoplasm in preparation for potential definitive Surgical Resection. From a Neurovascular perspective, we plan to follow-up in 3-6 months after he has completed his Cancer therapy, at which time we can confirm a durable cure of this extremely high-risk Cerebrovascular Pathology.
Discussion:
Pathophysiology:
Dural Arteriovenous Malformations (DAVM) or Arteriovenous Fistulas (DAVF) are pathological direct vascular shunts from the Dural Arteries to a Dural Venous Sinus or Cortical Vein. Most commonly, they occur independent of Intraparenchymal Pial Brain AVMS, but can sometimes be associated. Most commonly, Dural AVFs are found to be idiopathic, although occasionally can be found to be associated with craniotomy/surgery, trauma, or Dural Sinus Thrombosis. As opposed to Pial Brains AVMs, most DAVFs are often observed in older patients, and generally felt to be acquired or develop over time, the mean age of presentation in the 5th or 6th decade, equal prevalence by gender, although hemorrhage has been reported at a higher incidence in men. A genetic linkage or family history prevalence has not yet been identified.
Classification Systems:
Traditionally, there have been 2 widely used anatomic/angiographic classification systems used to describe Dural AVFs, primarily based on the pattern of venous drainage. The Cognard system was adapted from the original Djinjian system and classifies them based on the direction of venous drainage (antegrade or retrograde), the presence of Cortical Venous Drainage (CVD), and the architecture of the venous outflow. Similarly, the Borden-Shucart system classifies Dural AVFs on the site of venous drainage (Dural Sinus or Cortical Vein), the presence of CVD, and the number of fistula sites[1]. (Table 1) These original classification systems suggested that as Venous Drainage and CVD pattern progressed through these scales, the natural history risk of brain hemorrhage (ICH) or non-hemorrhagic neurologic deficits (NHND) increased.
Over the last decade, the neurovascular community has continued to research these patients, and made observations to suggest that the not only the presence of CVD, but the presenting symptoms of ICH or NHND may significantly affect the natural history of Dural AVFs. Zipfel etal. Proposed a new modified classification system which incorporated the presenting symptoms, which suggested a higher rate of ICH (7-8% annually) in patients presenting with CVD and ICH or NHND (termed Symptomatic CVD), than patients with Asymptomatic CVD patients who’s rate of hemorrhage in several retrospective studies was observed to be 1-2% annually (Table 2).1
CervicoMedullary AV Fistulas (Cognard Type 5):
Spinal Dural AVMs at this location represent a specifically challenging and aggressive special subtype of Dural arteriovenous shunts. These AV Fistulas may present as several subtypes based on their venous drainage patterns, located at the Surface of the spinal cord (SPAVF) or within the Dura mater of the nerve sleeve or leptomeninges (SDAVF).[2] Retrograde and Descending Spinal Perimedullary drainage patterns often present with progressive symptoms of myelopathy in the affected neural networks (as in our patient – dysarthria, hemiplegia, bowel/bladder dysfunction), while Antegrade/Ascending Intracranial Venous drainage into the Mesencephalic Veins and subsequent Petrosal and Intracranial Dural Sinuses is often associated with a higher risk of hemorrhagic presentation.[3]
Management Strategies and Treatment:
Patients presenting with Dural AVMs have a wide range of clinical presentations and may be managed with a variety of treatment strategies. Patients that present without symptoms, or manageable symptoms (ie. Low grade pulsatile tinnitus) and absence of CVD or high-grade features for hemorrhage may be managed conservatively and observed closely over time, clinically and with imaging (MRI, CT, or Angiography). If these patients develop new symptoms or demonstrate evidence of progression and develop high-risk features of CVD or aneurysm formation, then more aggressive treatment can be considered.
Many different therapeutic strategies have been successfully utilized to treat safely and effectively high-risk or symptomatic DAVMs, including at the Cervicomedullary junction. Transarterial embolization of the arterial-venous fistulas using coils and liquid embolic agents (NBCA, Onyx) may be used to successfully decrease the number and severity of the high-pressure A-V shunts, effectively reducing the risk of recurrent hemorrhage. In addition, in select cases, and Transvenous approach may be employed to access the anomalous venous structure to embolize the venous recipient of the fistulas and effect a cure. Transvenous embolization must be performed cautiously and in appropriate anatomic and clinical settings to ensure adequate collateral venous drainage. Microsurgical exposure and treatment thru Transcondylar and Suboccipital approaches has also been employed successfully, surgically obliterating the fistulas feeders and appropriate venous segments. Often, a multi-disciplinary approach has increasingly been used to personalize the treatment to each patient’s unique presentation.[4],[5]
References:
[1] Zipfel GJ, Shah MN, Refai D, Dacey RG Jr, Derdeyn CP. Cranial dural arteriovenous fistulas: modification of angiographic classification scales based on new natural history data. Neurosurg Focus. 2009;26(5):E14. doi:10.3171/2009.2.FOCUS0928
[2] Kai Y, Hamada J, Morioka M, Yano S, Mizuno T, Kuratsu J. Arteriovenous fistulas at the cervicomedullary junction presenting with subarachnoid hemorrhage: six case reports with special reference to the angiographic pattern of venous drainage. AJNR Am J Neuroradiol. 2005;26(8):1949-1954.
[3] Kinouchi H, Mizoi K, Takahashi A, Nagamine Y, Koshu K, Yoshimoto T. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg. 1998;89(5):755-761. doi:10.3171/jns.1998.89.5.0755
[4] Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194(3):671-680. doi:10.1148/radiology.194.3.7862961
[5] Fujimoto S, Takai K, Nakatomi H, Kin T, Saito N. Three-dimensional angioarchitecture and microsurgical treatment of arteriovenous fistulas at the craniocervical junction. J Clin Neurosci. 2018;53:140-146. doi:10.1016/j.jocn.2018.04.065
