Stroke and Carotid Disease
Title: Early Neurologic Decline in Tandem Carotid and MCA Occlusions
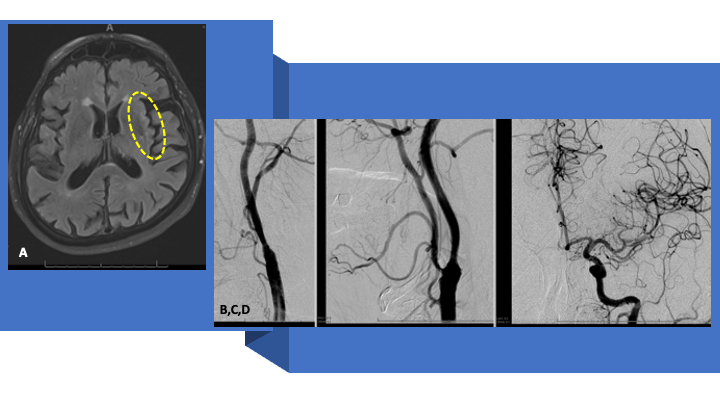
A 79-year-old man experienced an episode around 1030 PM while watching TV, when he had difficulty getting up from sitting, felt like he could not lift his legs to get up. Patient’s wife thought that his speech appeared garbled, and the patient felt concerned that he was having trouble getting words out. His wife also thought that he appeared to be drooling out of the right side of his mouth. Denied any facial droop and reports that she was looking for this symptom because she was concerned that he may be having a stroke. The symptoms resolved after 2 to 3 minutes. Patient also stated he had a slight headache. Denied neck pain, chest pain, or shortness of breath. Patient’s PMH included chronic kidney disease, hypertension, and hyperlipidemia. On presentation in the ED, he had returned to baseline (normal) with an NIHSS of 0 (1230 AM-630 AM). No IV tPA was administered. He was loaded with Plavix 300 mg, admitted for observation for a TIA and an MRI of the Brain was obtained (600 AM), demonstrating small diffusion positive ischemic injury in the left insular cortex (and lack of flow void in the left MCA) (Figure 1 A).

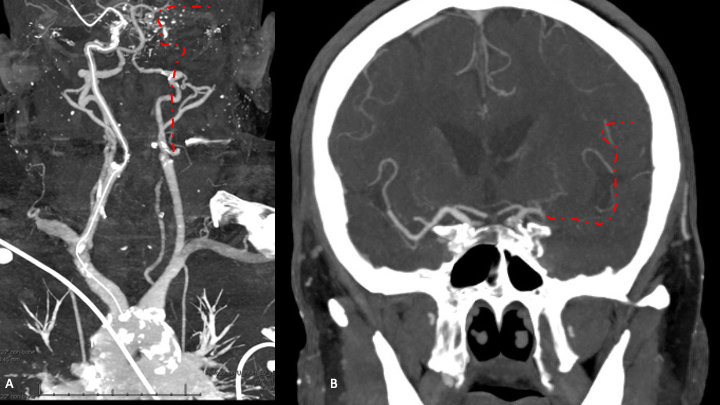
At approximately 6:30 AM as he was coming out of the MRI, the patient was noted to acutely progress to severely hemiparesis on the right side, with dysarthria, without aphasia or gaze preference (NIHSS 8). Emergent CTA and CTP were performed per our Stroke Protocols which demonstrate a large territory of tissue at risk in the left Frontal lobe MCA territory (Figure 1 B). CTA confirmed a complete occlusion of the left ICA at the Common Carotid Artery Bifurcation (Figure 2 A) with a tandem embolic occlusion of the M1 segment of the left MCA (Figure 2 B).
Figure 2:
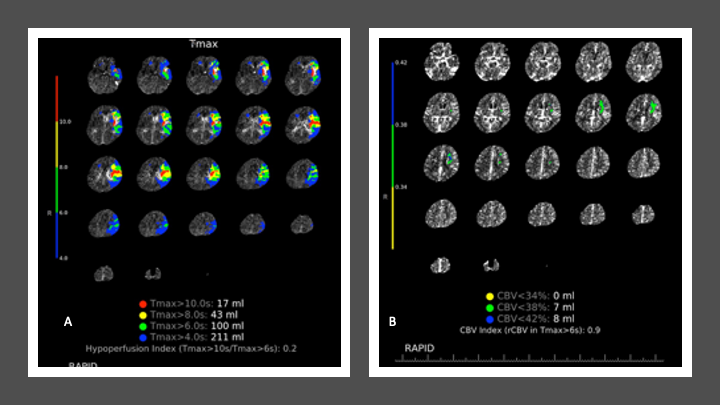
CT Perfusion reveals a large, mismatched territory of hypoperfusion of prolonged time to perfusion (TMax) and relatively preserved Cerebral Blood Volume (CBV), suggesting potentially salvageable penumbral ischemic brain injury (Figure 3 A and B).
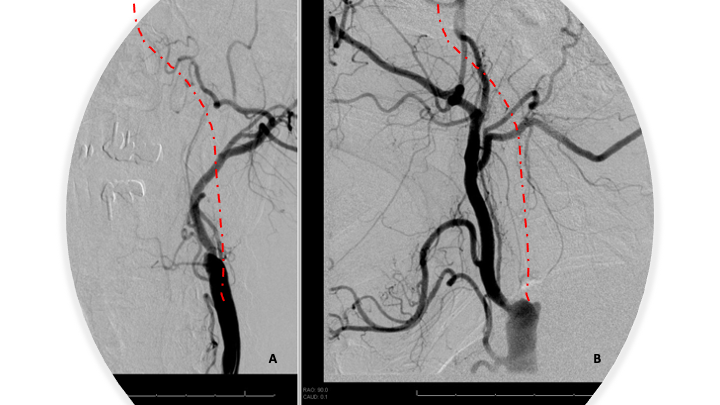
Given that there is an MRI showing acute stroke, unclear onset, Stroke Neurology decided to not proceed with IV thrombolysis (contra-indicated). Neuroendovascular Intervention was pursued based on the large vessel occlusions and favorable Cerebral Perfusion profile. A loading dose of ASA 600 mg PR was administered. Under MAC Anesthesia, an angiogram was performed confirming 100% occlusion of the left internal carotid artery at its origin with no antegrade opacification of the left intracranial circulation (Figure 4 A and B).
Figure 4:
Utilizing digital roadmap, a 0.035 Glidewire was used to probe the left ICA occlusion to establish a channel for further intervention. After several attempts, 0.035 Glidewire was successfully navigated through the occlusion. A 5 French diagnostic catheter was then carefully advanced through the left ICA occlusion over the wire to perform a careful “Dottering” on the left ICA occlusion to establish a channel for antegrade flow. The 6 French shuttle sheath was then advanced into the left ICA origin, but was not advanced across the stenosis secondary to resistance suggesting a very high-grade residual stenosis with possible underlying thrombus within the left ICA cervical bulb. At this point, aspiration thrombectomy was performed of the left ICA bulb with the 6 French shuttle, and direct aspiration using a 30-cc syringe. A small amount of thrombus was observed within the syringe, however a post initial thrombectomy angiogram demonstrates persistent occlusion of the left ICA. A Penumbra Jet7/3 max penumbra aspiration combination was then advanced over a Synchro 2 standard microwire and selective catheterization of the left ICA carotid bulb was performed with a Jet 7 catheter. Aspiration thrombectomy was then initiated a second time using the Jet 7 aspiration catheter and penumbra aspiration system for approximately 3 to 5 minutes, after which the thrombectomy catheter was carefully removed with negative aspiration of the guiding catheter/shuttle sheath. Post thrombectomy angiogram demonstrates very high-grade near complete occlusion persisting with very minimal antegrade perfusion through the left carotid bulb. We then decided to proceed with emergent angioplasty of this left ICA occlusion in an effort to establish antegrade flow and a pathway for intracranial navigation and thrombectomy. Utilizing digital roadmap, a Viatrac 4 x 40 PTA balloon was primarily navigated over a Synchro 2 standard microwire across the left ICA stenosis. After being positioned across the lesion, the balloon was gradually inflated over approximately 30 seconds to 14 atm until waisting of the balloon was observed. Post angioplasty angiography demonstrates significant improvement in the left ICA occlusion with approximately 60 to 70% residual stenosis however brisk antegrade flow into the left intracranial circulation. Angiography of the left intracranial ICA circulation confirms persistent occlusion of the proximal left M1 MCA segment (Figure 5 A and B).
Figure 5:
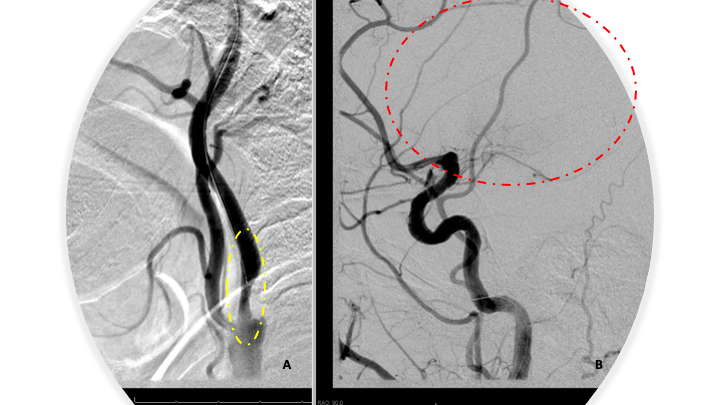
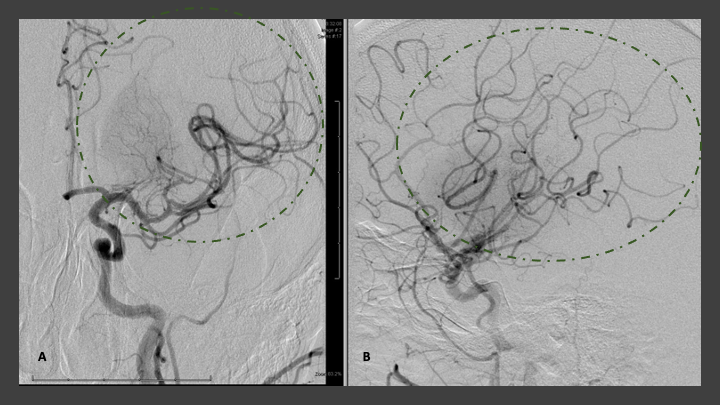
At this point, the Jet 7/3 max penumbra aspiration system was then reinserted and carefully navigated across the residual high-grade stenosis of the left ICA. Utilizing digital roadmap, the left MCA M1 segment occlusion was traversed with the microwire, followed by the 3 max aspiration catheter, followed by placement of the Jet 7 aspiration catheter within the thrombus within the proximal left M1 segment. Aspiration thrombectomy of this segment was then performed for approximately 3 to 5 minutes until retrograde flow was observed within the tubing. There did appear to be a large fragment of thrombus that was withdrawn into the tubing and into the canister suggesting potential revascularization. The Jet 7 aspiration catheter was then removed under negative pressure at the guiding catheter/sheath level. Post thrombectomy angiogram demonstrates complete revascularization of the left MCA representing TICI 3 complete reperfusion with no distal branch occlusions observed (Figure 6 A and B). (Trade names were included, but only for specific reference to devices used in this operation. No specific advocacy is implied, and many comparable devices may and have been utilized based on operator preferences and device availability)
Figure 6:
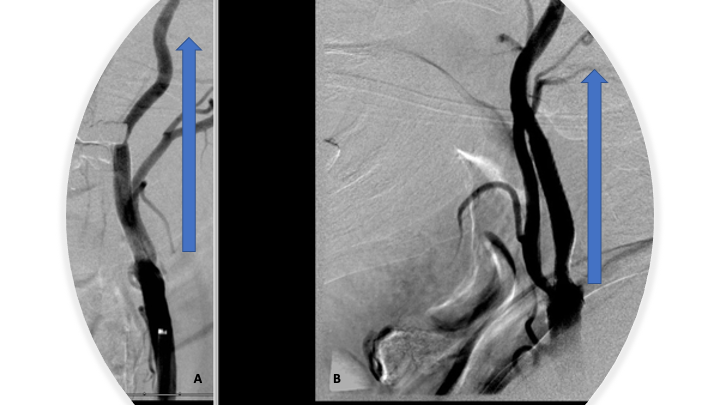
Moderate hyperemia was noted within the basal ganglia compatible with post ischemic changes. The blood pressure was reduced to less than 140/90 following revascularization to minimize the risk of reperfusion injury or hemorrhage. Angiography of the left common carotid/internal carotid artery stenosis was once again performed continue to demonstrate approximately 70% residual stenosis at the site initial occlusion following angioplasty. At this point, the embolic protection filter was then carefully navigated across the stenosis into the distal cervical segment of the left ICA. The embolic protection filter was then deployed and maintained in position. An Acculink 10–7 x 40 mm carotid stent was then carefully navigated across the stenosis over the embolic protection filter and positioned within the proximal cervical left ICA and distal left common carotid artery and carefully deployed across the stenosis. Post stent deployment angiography demonstrates significant improvement in the stenosis now approximately 30-40% residual stenosis with brisk antegrade filling of the left MCA and intracranial ICA circulation (Figure 7 A and B).
Figure 7:
Intracranial angiograms confirmed complete revascularization without any branch occlusions. Patient remained hemodynamically stable during this emergency procedure without symptomatic bradycardia or hypotension following angioplasty or stenting. Immediate postoperative neurologic examination demonstrates complete reversal of the right-sided hemiparesis with absence of drift, facial symmetry, fluent speech, language comprehension, visual fields (NIHSS 0). He made a complete recovery and was cleared for discharge home with home care with no deficits, MRS 0, 2 days after intervention.
Over the last 6 months, he continues to do very well having return to all his activities of daily living with no limitations. He denies any issues with language, vision, lateralizing weakness, speech, swallowing, balance, or gait. Followup MRI and angiography were performed at 3 months, demonstrating no significant infarcts (including prior diffusion positive insula-reversed!!!) and further passive expansion of the self-expanding stent with no significant residual stenosis (<10% per NASCET) at the Carotid Stent, and brisk filling the left ICA intracranial circulation (Figure 8 A and B).
Figure 8:
Discussion:
Many acute stroke patients present with transient, fluctuating, or minor (NIHSS<5) neurologic deficits. Patients without a Large Vessel Occlusion (LVO)(ICA, MCA, Basilar) generally have favorable outcomes, with only 3.1% (55/1749) experiencing Early Neurologic Deterioration (END, >4pts NIHSS) in the SITS International Stroke Registry of 2553 patients analyzed.[1] However, in the presence of an LVO, END was observed in 30% of patients with a terminal ICA or tandem occlusions (ICA+MCA); 17% in extracranial ICA occlusions; and 9% of Proximal M1 MCA occlusions in the same study. Many similar studies of mild presentations (<NIHSS 5) and LVO have similar observation of END ranging from 20-40%.[2],[3] Common mechanisms felt to contribute to Early Neurologic Deterioration include failure of collateral circulation, extension of the thrombus, or secondary thromboembolism. Advanced Perfusion Imaging (CTP or MRP) and MRI Diffusion may help predict which patients are more likely to progress, with larger hypoperfusion and mismatch volumes observed in patients experiencing END.[4]
Our patient presented with a Tandem (ICA + MCA) occlusion, which can be found in as many as 20% of stroke patients. This sub-type of anterior circulation LVO’s responds very poorly to IV-tPA alone, with only 22.7% improving > 4 pts. and only 18.2% achieving functional independence at 3 months (mRS<3).[5] As Neuroendovascular Therapy has gained more widespread acceptance and experience, many Neurointerventionalists commonly employ a combination of Emergent PTA and Stenting in combination with Intracranial Mechanical Thrombectomy for these critically ill patients. In an early study of 170 patients treated at 4 German Stroke Centers, successful revascularization to TICI 2b or better (>50% reperfusion) was achieved in 77%, in-hospital Mortality of 19%, Favorable mRS < 3 in 36%, and Hemorrhage Transformation in 9% of patients.[6] More recent experiences include the Thrombectomy in Tandem Lesion registry (TITAN) have confirmed improved outcomes with Combined Stenting and Thrombectomy with or without tPA with 90d favorable outcomes in 51-62% of their patient cohorts.[7]
The optimal technical approach and treatment strategies for these scenarios remain controversial. Which lesion to treat first (intracranial vs. extracranial), angioplasty or stenting (primary approach), and anti-platelet/anti-coagulation regimens to be employed? Proponents of the extracranial first favor collateral augmentation, while intracranial first favor primary revascularization. PTA alone proponents favor limiting anti-platelet hemorrhagic risks, while stenting proponents favor definitive treatment and limiting risks of re-occlusion. A recent systematic review and meta-analysis of 33 studies found no significant difference in good outcomes or safety independent of approach utilized, with approximately 50% of patients having good neurological outcomes in their subgroup analysis.[8]
In conclusion, Tandem occlusions and Fluctuating Low NIHSS stroke syndromes represent challenging high-risk scenarios with potential for devastatingly poor outcomes. These patients should be managed cautiously and may be strongly considered for early intervention, especially if presenting with proximal LVO’s or evidence of perfusion failure on advanced imaging. Combination approaches of mechanical thrombectomy and Carotid PTA and Stent may result in favorable independent outcomes in as many as 1 out of 2 patients if performed early.
References:
[1] Mazya MV, Cooray C, Lees KR, et al. Minor stroke due to large artery occlusion. When is intravenous thrombolysis not enough? Results from the SITS International Stroke Thrombolysis Register. Eur Stroke J. 2018;3(1):29-38. doi:10.1177/2396987317746003
[2] Saleem Y, Nogueira RG, Rodrigues GM, et al. Acute Neurological Deterioration in Large Vessel Occlusions and Mild Symptoms Managed Medically. Stroke. 2020;51(5):1428-1434. doi:10.1161/STROKEAHA.119.027011
[3] Lee VH, Thakur G, Nimjee SM, et al. Early neurologic decline in acute ischemic stroke patients receiving thrombolysis with large vessel occlusion and mild deficits. J Neurointerv Surg. 2020;12(11):1085-1087. doi:10.1136/neurintsurg-2020-015871
[4] Gwak DS, Kwon JA, Shim DH, Kim YW, Hwang YH. Perfusion and Diffusion Variables Predict Early Neurological Deterioration in Minor Stroke and Large Vessel Occlusion. J Stroke. 2021;23(1):61-68. doi:10.5853/jos.2020.01466
[5] Rubiera M, Ribo M, Delgado-Mederos R, et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke. 2006;37(9):2301-2305. doi:10.1161/01.STR.0000237070.80133.1d
[6] Behme D, Mpotsaris A, Zeyen P, et al. Emergency Stenting of the Extracranial Internal Carotid Artery in Combination with Anterior Circulation Thrombectomy in Acute Ischemic Stroke: A Retrospective Multicenter Study. AJNR Am J Neuroradiol. 2015;36(12):2340-2345. doi:10.3174/ajnr.A4459
[7] Anadani M, Spiotta AM, Alawieh A, et al. Emergent Carotid Stenting Plus Thrombectomy After Thrombolysis in Tandem Strokes: Analysis of the TITAN Registry. Stroke. 2019;50(8):2250–2252. doi:10.1161/STROKEAHA.118.024733
[8] Wilson MP, Murad MH, Krings T, et al. Management of tandem occlusions in acute ischemic stroke – intracranial versus extracranial first and extracranial stenting versus angioplasty alone: a systematic review and meta-analysis. J Neurointerv Surg. 2018;10(8):721-728. doi:10.1136/neurintsurg-2017-013707
