Brain AVM - Ruptured Dural AVF
55 yo woman develops sudden severe headache and near loss of consciousness
A 55-year-old woman presented with acute onset of severe headache, and possible witnessed seizure. Imaging confirmed intraventricular hemorrhage primarily focused within the fourth ventricle with diffuse subarachnoid hemorrhage within the posterior fossa and along the tentorium as observed on non Contrast CT and MRI of the Brain(Figure 1).
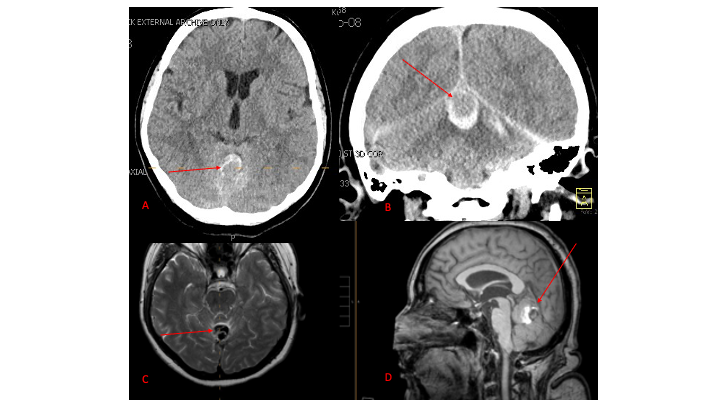
Figure 1. A and B) Non-Contrast CT Axial and Coronal demonstrate large ruptured Varix/Aneurysm along the
Incisura of the posterior fossa which appears to have mixed hemorrhagic components and enhancement on MRI T2
and post contrast imaging (C and D)
No severe neurologic deficits were observed initially. Her cerebral angiogram revealed a high flow Dural arterial venous fistulous malformation of the tentorium supplied by the bilateral external carotid and left vertebral arteries into a large venous varix within the fourth ventricle at the site of the hemorrhage. Deep venous drainage is observed into the Galenic and Straight Sinus of the posterior fossa. (Figure 2)
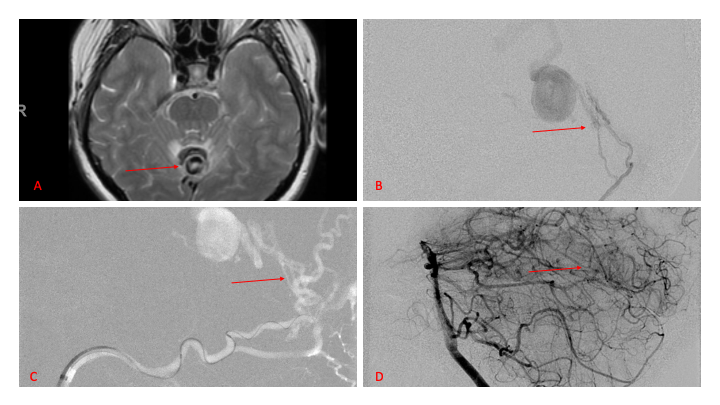
Figure 2. [A] Ruptured Varix/Aneurysm of the Deep Venous Posterior Fossa drainage and AV shunting (arrows) [B] Posterior Meningeal Artery Fistula [C] Right Middle Meningeal Artery Fistula [D] Post embolization Left Vertebral Angiogram demonstrates resolution of the high flow and pressure A-V Shunting following successful embolization with liquid embolic (NBCA).
This patient’s clinical presentation with brain hemorrhage, large aneurysm, and high-pressure shunts is generally considered high risk for recurrent hemorrhage and associated morbidity. We proceeded to perform embolization of the Left Posterior Meningeal Artery (Figure 2 B and D), and the Bilateral External Carotid Artery Middle Meningeal and Occipital Arteries (Figures 3 and 4) using liquid embolic agents (N-butyl Cyanoacrylate) penetrate the distal branches of the fistulas and A-V shunts.

Figure 3. Right ECA AP and Lateral angiograms Pre (A and B) and Post NBCA embolization (C and D) demonstrate near complete obliteration of right Occipital and Middle Meningeal Artery fistulas
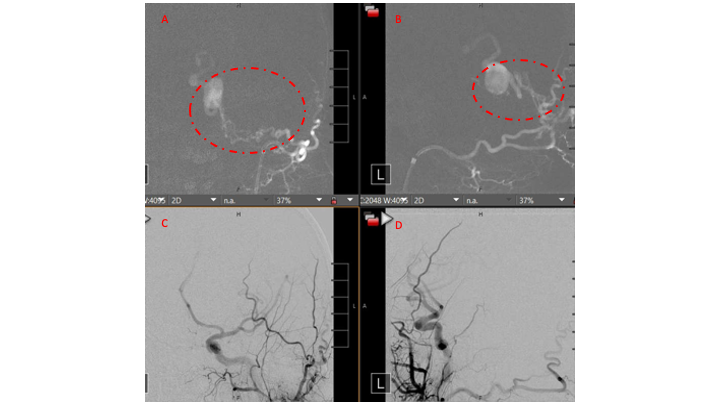
Figure 4. Left ECA AP and Lateral angiograms Pre (A and B) and Post NBCA embolization (C and D) demonstrate near complete obliteration of Left Occipital Artery Fistulas
Embolization was successful at immediately reducing the pressure into the Aneurysmal Varix with greater than 90% reduction in A-V shunts. After an intensive period of critical care monitoring and care for her initial brain hemorrhage, she made a near complete recovery with mild intermittent left sided paresthesias and generalized deconditioning. After receiving inpatient and outpatient Acute Rehabilitation, Physical, and Occupational therapy, she completely recovered all of her Activities of Daily Living (ADLs) at 3 months and achieved an modified Rankin Scale (mRS) of 0. On follow-up angiography, small residual low flow A-V shunts were identified and targeted for Gamma Knife Radiosurgery. (Figure 5 and 6)
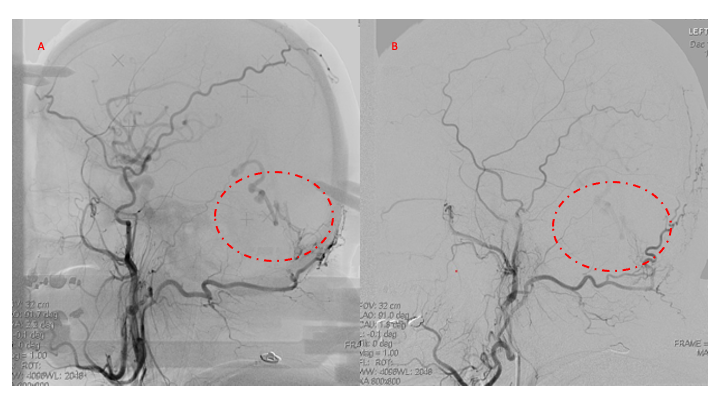
Figure 5. Bilateral ECA Lateral angiograms (A and B) demonstrates small residual indirect supply to the AVM from the Bilateral Occipital arteries.
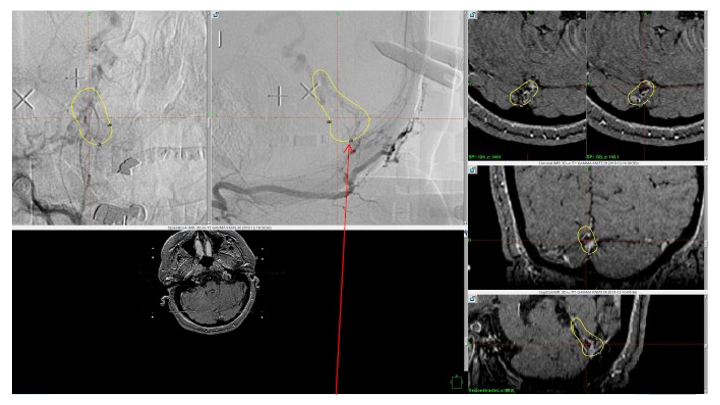
Figure 6. MRI and Catheter Angiograms precisely localize the residual A-V shunts vessels to perform Gamma-Knife Radiosurgery to deliver therapeutic doses to the targets.
Over the 12 to 18 months, she continued to remain event-free and maintain her active independent lifestyle. Delayed angiography over that period confirmed complete obliteration of the Left ECA and Vertebral Artery shunts with near complete obliteration of the Right ECA shunts. (Figure 7)
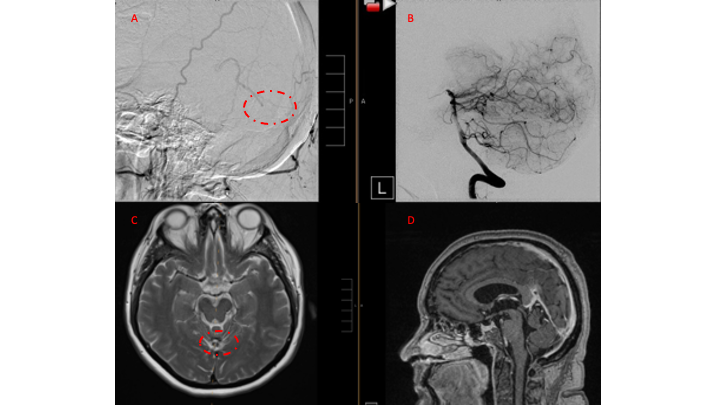
Figure 7. 12 mos follow-up angiograms confirm complete obliteration of the left ECA and Vertebral
Supply to the AVM, with a small late-filling component from the right Occipital artery that is markedly
Reduced in size and flow (top row). Near complete resolution of the aneurysm and hematoma
Is observed on 16 mos post Gamma Knife MRI (bottom row).
Discussion:
Pathophysiology:
Dural Arteriovenous Malformations (DAVM) or Arteriovenous Fistulas (DAVF) are pathological direct vascular shunts from the Dural Arteries to a Dural Venous Sinus or Cortical Vein. Most commonly, they occur independent of Intraparenchymal Pial Brain AVMS, but can sometimes be associated. Most commonly, Dural AVFs are found to be idiopathic, although occasionally can by found to be associated with craniotomy/surgery, trauma, or Dural Sinus Thrombosis. As opposed to Pial Brains AVMs, most DAVFs are often observed in older patients, and generally felt to be acquired or develop over time, the mean age of presentation in the 5th or 6th decade, equal prevalence by gender, although hemorrhage has been reported at a higher incidence in men. A genetic linkage or family history prevalence has not yet been identified.
Classification Systems:
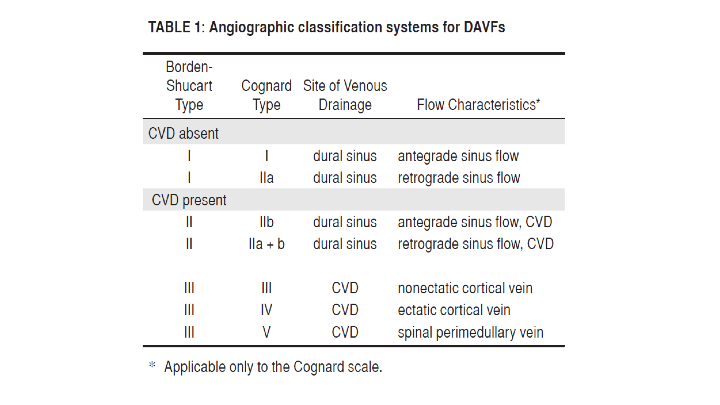
Traditionally, there have been 2 widely used anatomic/angiographic classification systems used to describe Dural AVFs, primarily based on the pattern of venous drainage. The Cognard system was adapted from the original Djinjian system and classifies them based on the direction of venous drainage (antegraded or retrograde), the presence of Cortical Venous Drainage (CVD), and the architecture of the venous outflow. Similarly, the Borden-Shucart system classifies Dural AVFs on the site of venous drainage (Dural Sinus or Cortical Vein), the presence of CVD, and the number of fistula sites. (Table 1)
These original classification systems suggested that as Venous Drainage and CVD pattern progressed through these scales, the natural history risk of brain hemorrhage(ICH) or non-hemorrhagic neurologic deficits(NHND) increased.
(From: Zipfel etal. Neurosurg Focus 26 (5):E14, 2009)
Over the last decade, the neurovascular community has continued to research these patients, and made observations to suggest that the not only the presence of CVD, but the presenting symptoms of ICH or NHND may significantly affect the natural history of Dural AVFs. Zipfel etal. Proposed a new modified classification system which incorporated the presenting symptoms, which suggested a higher rate of ICH (7-8% annually) in patients presenting with CVD and ICH or NHND (termed Symptomatic CVD), than patients with Asymptomatic CVD patients who’s rate of hemorrhage in several retrospective studies was observed to be 1-2% annually (Table 2).

Key Learning Points:
- Indirect Dural AV Fistula Vascular Malformations represent high risk lesions that result from acquired and/or congenital indirect fistula and A-V shunts that can arise within many of the deep venous structures of the brain (Superficial and Deep Venous Drainage systems).
- Many patients may present with initially mild symptoms of pulsatile tinnitus or headaches but may progress over time to more severe high-risk symptomatology including hemorrhagic stroke, permanent neurologic deficits, and even mortality.
- Embolization is a well-established safe and effective treatment to reduce or obliterate Dural A-V Fistulas and AVMs to reduce the risk of recurrent hemorrhage and associated morbidity
- Gamma Knife Radiosurgery has demonstrated successful and durable treatment of many Brain AVM’s when effectively selected and targeted.
- Early evaluation by an expert multi-disciplinary team is critical to diagnosis, management, and successful treatment and recovery with a variety of medical, endovascular, and surgical approaches.
References:
- Lewis AI, Tomsick TA, Tew JM Jr. Management of tentorial dural arteriovenous malformations: transarterial embolization combined with stereotactic radiation or surgery. J Neurosurg. 1994;81(6):851-859. doi:10.3171/jns.1994.81.6.0851
- Chen CJ, Lee CC, Ding D, et al. Stereotactic radiosurgery for intracranial dural arteriovenous fistulas: a systematic review. J Neurosurg. 2015;122(2):353-362. doi:10.3171/2014.10.JNS14871
- Starke RM, McCarthy DJ, Chen CJ, et al. Evaluation of stereotactic radiosurgery for cerebral dural arteriovenous fistulas in a multicenter international consortium. J Neurosurg. 2019;132(1):114-121. doi:10.3171/2018.8.JNS181467
- Zipfel GJ, Shah MN, Refai D, Dacey RG Jr, Derdeyn CP. Cranial dural arteriovenous fistulas: modification of angiographic classification scales based on new natural history data. Neurosurg Focus. 2009;26(5):E14. doi:10.3171/2009.2.FOCUS0928
